Some may assume that their winery operation may be too small to get inspected by the FDA, in relation to the 2011 Food Safety and Modernization Act (FSMA) regulation. Is this really the case?
The FDA now publicizes many of the food and beverage establishments that have been inspected to date, as well as the outcome of the inspection, through the Classifications Database. Outcome classifications are broken down by the following three results:
- NAI: No Action Indicated, indicating that “no objectionable conditions or practices were found during the inspection,”
- VAI: Voluntary Action Indicated, indicating “objectionable conditions or practices were found but the agency is not prepared to take or recommend any administrative or regulatory action,” and
- OAI: Official Action Indicated, indicating “regulatory and/or administrative actions will be recommended.”
This language, as well as further information about the Classifications Database, can be found on their FAQ page.
Are Wineries in My State Getting Inspected?
While the FDA Classifications Database does not include all FDA inspections, I took the time to sift through their available data and look at 10 individual states.
By using the search terms: “wine,” “winery,” and “vineyard,” I was able to review how many wineries have been inspected in various states and recorded within the database. I also made sure to eliminate any wine importers or distributors that may have appeared on the list. These numbers are valid through April 2019. Please note that breweries, [hard] cideries, and distilleries are also subject to the FSMA regulation and FDA inspections, but are not included in the table below.
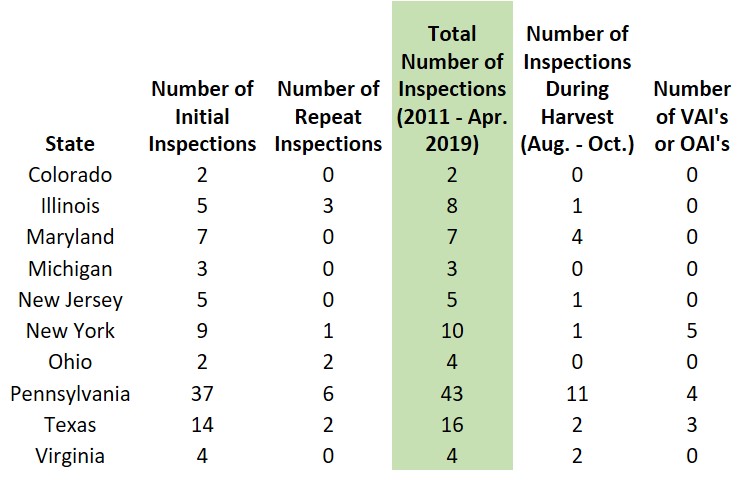
Is your state not on the list?
This does not mean your state’s wineries are not getting inspected. It simply means I did not sift through the database for that particular state. FSMA is a federal regulation that applies to all U.S. wineries.
Is Preparing for FSMA Worth it?
In case you think a low number of inspections for your state is an indication that you do not need to complete your FDA documentation, I’d caution you in this rationale.
It’s unclear why some data may be missing from the FDA Classification Database. With that in mind, it’s possible more winery inspections could be taking place in your state of business. However, that data may be missing from the database system.
Furthermore, many state regulating agencies are now getting trained on, or in the beginning stages of, conducting FSMA inspections on the FDA’s behalf. Since wineries and most alcohol-producing facilities are considered “low risk” manufacturing facilities, it’s possible that a state inspector will provide an inspection instead of an FDA inspector.
Finally, it’s important to remember that many wineries are already practicing current good manufacturing practices (cGMPs) and have some small employee training programs. Having these two entities is the bulk of the work affiliated with FSMA. Getting it down on paper to share with an inspector is the next step.
There’s a fair argument that taking the time to create your documentation can actually improve inter-personnel communication, operations understanding, and production efficiency. The documentation helps streamline your processes and procedures, making them easily accessible and understandable by all employees. Think about it. It saves YOU a lot of time to provide a standard operating procedure (SOP) to your employees, have them work through it, and then come to you with questions, as opposed to you always needing to be present for that operational task.
You Can Start Preparing for an FDA Inspection Today
There’s still time for you to get in the FSMA spirit with the DG Winemaking 5-day email course “Getting Your Winery Ready for an FDA Inspection.” It’s a free course in which we’ll review some of the key documentation required by wineries to obtain FDA compliance and help them get through their inspections with ease.
With registration for June 17 – 21, 2019 email course, you’ll also receive a free, downloadable checklist: Is Your Winery FSMA Ready? This checklist goes step-by-step through necessary actions in creating your documentation that an FDA inspector will look for during an inspection.
Want your free copy of the Is Your Winery FSMA Ready? checklist and free enrollment in our 5-day email course?
